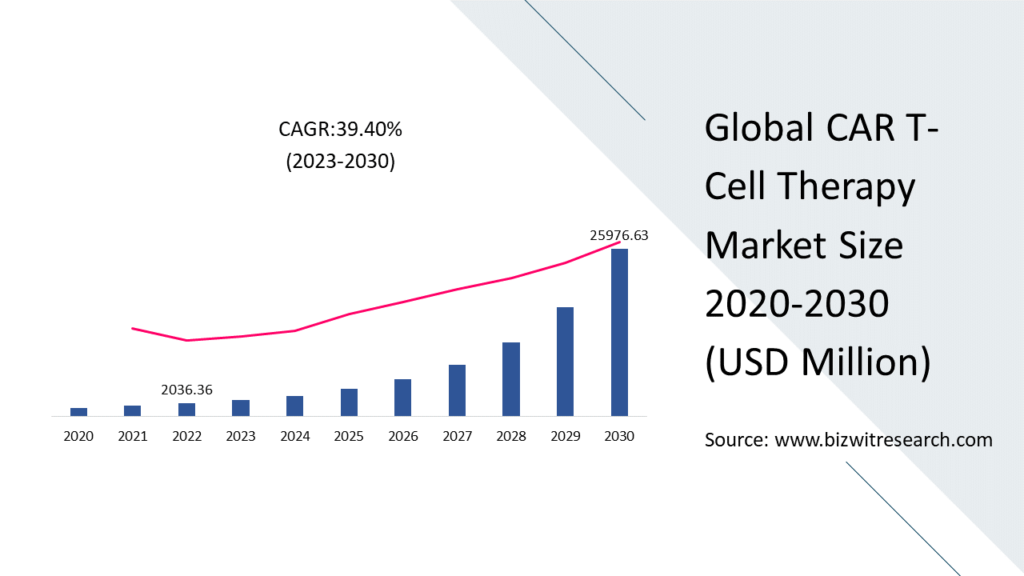
Global CAR T-Cell Therapy Market is valued at approximately USD 2036.36 million in 2022 and is anticipated to grow with a healthy growth rate of more than 39.40% over the forecast period 2023-2030. Chimeric antigen receptor (CAR) T cell therapy is a novel form of cancer therapy in which medical experts modify T cells in a lab before injecting them into cancer patients’ bodies to enable them to detect and eliminate cancer cells. The immune system is trained to recognize malignant cells by CAR T-cell therapy. The immune response against malignant cells is boosted by CAR T-cell treatment.
The market for CAR T-cell therapy is primarily driven by an increase in the demand for suitable therapeutics for the treatment of cancer, favorable reimbursement policies for CAR T-cell therapy medications in some countries, and an increase in consumer awareness of CAR T-cell therapy medications. Additionally, the recent commercialization of CAR-T cell therapy and the surging clinical trial activities are further influencing the market expansion.

For further analysis on the study, request a sample copy
In addition, an increase in the incidence of cancer such as leukemia and lymphoma are directly augmenting the demand for CAR T-Cell therapy. According to World Health Organization (WHO), in 2020, the number of leukemia cancer cases is estimated to account for 475 thousand, which is projected to reach 692 thousand patients by the year 2040. Accordingly, the growing prevalence of cancer is fueling the demand for a wide range of diagnosis and treatment alternatives, which, in turn, accelerates the growth of the CAR T-Cell therapy Market.
Moreover, the growing investment in research and development, as well as increasing advancements in technology present various lucrative opportunities over the forecasting years. However, the unavailability of skilled professionals and the high cost of developing CAR T-cell therapy are challenging the market growth throughout the forecast period of 2023-2030.
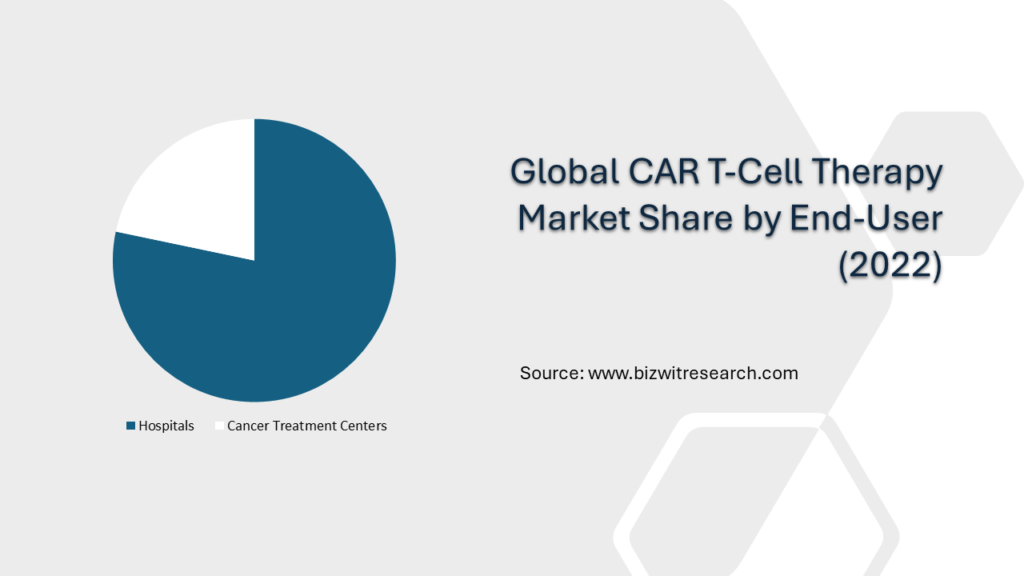
End-User Overview
By End User, the market is studied under Hospitals and Cancer Treatment Centers. Among these Hospitals dominate the market with 78.28% share in 2022 owing to factors such as the increasing investment in the development of cancer screenings and treatments within hospital settings. Additionally, the Cancer Treatment Centers segment is growing with the highest CAGR during 2023-2030 due to the rising initiatives and investment for the development of cancer treatment.

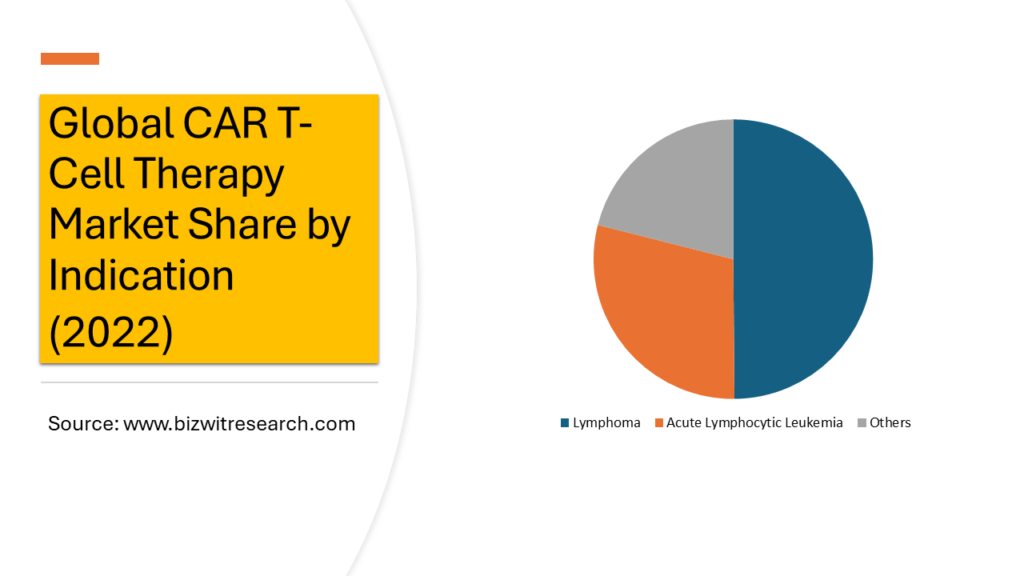
Indication Overview
By Indication, the market is studied under Lymphoma, Acute Lymphocytic Leukemia and Others. Among these Lymphoma dominates the market with 49.87% share in 2022. Lymphoma, characterized by the abnormal growth of lymphocytes, has been a key target for CAR T-cell therapy. It includes various subtypes, such as B-cell lymphomas and T-cell lymphomas, each posing unique challenges and opportunities for treatment.

Drug Type Overview
By Drug Type, the market is studied under Axicabtagene Ciloleucel, Tisagenlecleucel, Brexucabtagene Autoleucel and Others. Among these, Axicabtagene ciloleucel dominates the market with 39% market share in 2022. Axicabtagene ciloleucel, commonly known as Yescarta, is a chimeric antigen receptor T-cell (CAR T-cell) therapy designed to treat certain types of blood cancers, particularly B-cell lymphomas.
Regional Overview
Global CAR T-Cell Therapy Market is studied under 5 major regions, namely, North America, Asia Pacific, Europe, Latin America and Middle East & Africa. North America stands as the dominating region in the global CAR T-cell therapy market, driven by advanced healthcare infrastructure, robust research and development activities, and early regulatory approvals. The presence of major pharmaceutical companies and academic institutions engaged in cutting-edge CAR T-cell research further strengthens the market. A well-established reimbursement framework and high awareness among healthcare professionals and patients contribute to the widespread adoption of CAR T-cell therapies in treating hematologic malignancies.

Asia Pacific emerges as the fastest-growing region in the global CAR T-cell therapy market, fueled by a large patient population, rising cancer prevalence, and increasing healthcare investments. Improving regulatory frameworks and a surge in clinical trials contribute to the rapid growth. Countries like China and Japan play pivotal roles in advancing CAR T-cell therapy research and development.
Major market players included in this report are:
Autolus Therapeutics
Bluebird bio, Inc.
Bristol-Myers Squibb
Caribou Biosciences, Inc.
Cartesian Therapeutics, Inc.
PeproMene Bio., Inc.
Gilead Sciences, Inc. (Kite Pharma Inc.)
Merck & Co., Inc.
Novartis AG
Amgen Inc.
Recent Developments in the Market:
Ø In June 2022, Bristol-Myers Squibb Company announced that the company received the U.S. Food and Drug Administration (FDA) approval for Breyanzi CAR T cell therapy to cure refractory or relapsed large B-cell lymphoma after one prior therapy. The Phase 2 pilot company-sponsored research of a CAR T cell treatment in patients with primary refractory or relapsed LBCL served as the foundation for the approval.
Ø In February 2022, Johnson & Johnson Services, Inc. declared that the U.S. Food and Drug Administration had approved CARVYKTI (ciltacabtagene autoleucel), a BCMA-Directed CAR-T Immunotherapy for the treatment of Patients with Relapsed or Refractory Multiple Myeloma.
Global CAR T-Cell therapy Market Report Scope:
ü Historical Data – 2020 – 2021
ü Base Year for Estimation – 2022
ü Forecast period – 2023-2030
ü Report Coverage – Revenue forecast, Company Ranking, Competitive Landscape, Growth factors, and Trends
ü Segments Covered – Indication, Drug type, End user, Region
ü Regional Scope – North America; Europe; Asia Pacific; Latin America; Middle East & Africa
ü Customization Scope – Free report customization (equivalent up to 8 analyst’s working hours) with purchase. Addition or alteration to country, regional & segment scope*
The objective of the study is to define market sizes of different segments & countries in recent years and to forecast the values to the coming years. The report is designed to incorporate both qualitative and quantitative aspects of the industry within countries involved in the study.
The report also caters detailed information about the crucial aspects such as driving factors & challenges which will define the future growth of the market. Additionally, it also incorporates potential opportunities in micro markets for stakeholders to invest along with the detailed analysis of competitive landscape and product offerings of key players. The detailed segments and sub-segment of the market are explained below:
By Indication:
Lymphoma
Acute Lymphocytic Leukemia
Others
By Drug type:
Axicabtagene Ciloleucel
Tisagenlecleucel
Brexucabtagene Autoleucel
Others
By End-user:
Hospitals
Cancer Treatment Centers
By Region:
North America
U.S.
Canada
Europe
UK
Germany
France
Spain
Italy
ROE
Asia Pacific
China
India
Japan
Australia
South Korea
RoAPAC
Latin America
Brazil
Mexico
Middle East & Africa
Saudi Arabia
South Africa
Rest of Middle East & Africa